1. Self-reporting and self-adjusting LCD
(Self-reporting and self-regulating liquid crystals)
Material Name: LCD
Research Team: Abbott Research Group, University of Wisconsin-Madison
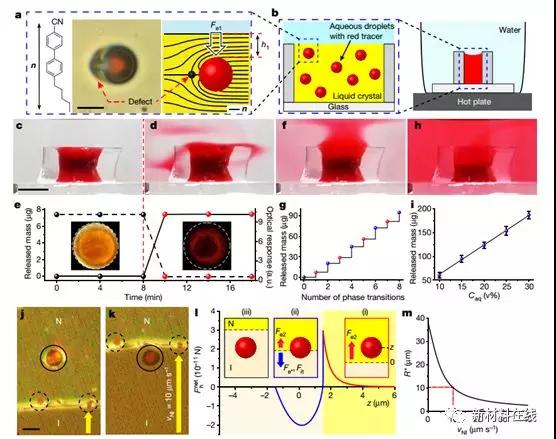
Liquid crystal (LC) is an anisotropic fluid that combines the long-range order of the crystal with the fluidity of the liquid. These features have been combined to create reconfigurable materials that can optically report their environmental information, such as electric field changes (smartphone displays), temperature changes (thermometers) or mechanical shear changes, and chemical and biological stimuli. (sensor), etc. But there is still an unmet need for responsive materials that not only need to convey their environmental information, but also transform the environment through self-regulating chemical interactions. Kim et al. demonstrated a series of stimuli that can trigger a pulse (transient) or a continuous release of micro-goods (aqueous droplets or solid particles and their chemical contents) in the LC at the beginning. The liquid crystal material is capable of self-reporting and self-regulation in a chemically responsive manner to the target physical, chemical, and biological events, and the specific response can be achieved by elastic, electric double layers in different geometries (eg, wells, membranes, and droplets). The interaction of buoyancy and shear forces is pre-programmed to achieve. These LC materials have the ability to exceed conventional materials for controlled microcarrier release and can perform complex functions such as optically reporting stimuli (eg, mechanical shear stress generated by moving bacteria) and then passing through a feedback loop The self-regulating mode responds (eg, releases the minimum amount of biocide required to cause bacterial cell death). (Nature DOI: 10.1038/s41586-018-0098-y)
2. The atomic cause of water vapor to promote oxidation of the alloy
(Atomic origins of water-vapour-promoted alloy oxidation)
Material Name: Nickel-chromium alloy
Research Team: Chongmin Wang Research Group, Pacific Northwest National Laboratory, USA
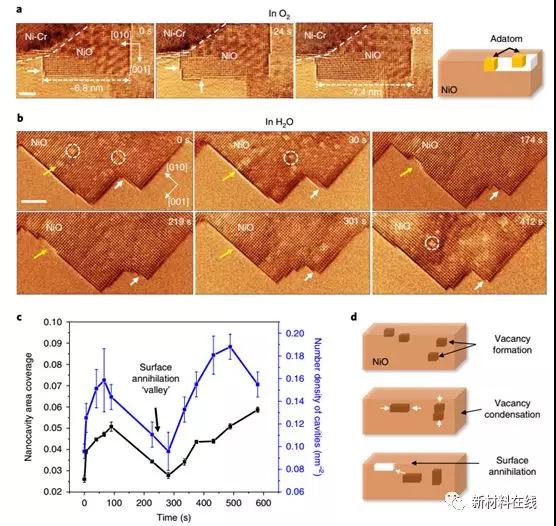
For many material applications such as steam generators, turbine engines, fuel cells, catalysts and corrosion, or the presence of water vapor, either intentional or unavoidable, is very important. Phenomenologically, it has been noted that water vapor accelerates the oxidation of metals and alloys. But the atomic mechanism behind this oxidation is still unknown. Luo et al. revealed by direct in-situ atomic-scale transmission electron microscopy and density functional theory calculations that the water vapor enhanced oxidation of nickel-chromium alloys is related to the formation, migration and aggregation of cation and anion vacancies promoted by proton lysis. The protons produced by the hydrolysis can occupy the interstitial sites in the oxide lattice, thereby reducing the vacancy formation energy and reducing the diffusion barrier of the cations and anions, which leads to an increase in oxidation in a humid environment with an elevated temperature. This work provides new insights into the oxidation of water vapor-enhanced alloys and has a major impact on other materials and chemical processes involving water vapor, such as corrosion, heterogeneous catalysis and ion conduction. (Nature Materials DOI: 10.1038/s41563-018-0078-5)
3. Energy band structure engineering of 2D materials using patterned dielectric superlattices
(Band structure engineering of 2D materials using patterned dielectric superlattices)
Material Name: Graphene
Research Team: Dean Research Group, Columbia University, USA
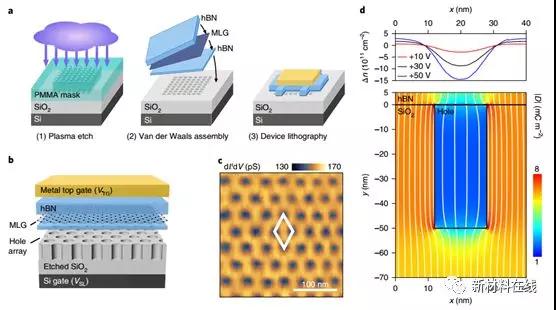
The use of external electric fields to process electrons in two-dimensional materials provides a way for integrated energy band engineering. By applying an artificially designed spatial periodic superlattice potential, the electronic properties can be further altered beyond the limits of naturally occurring atomic crystals. Forsythe et al. reported a new approach to fabricating high mobility superlattice devices by patterning surface dielectrics and integrating them with atomically thin van der Waals materials. They solved the tough trade-off between device handling and mobility reduction in super-lattice engineering in traditional systems through separate device assembly and superlattice fabrication processes. The improved electrostatics of atomic-scale ultra-thin materials make it possible to have super-lattice patterns smaller than the wavelengths of the previous examples. In addition, Forsythe et al. observed the formation of a replica Dirac cone in a ballistic graphene device with a superlattice below 40 nm and reported fractals from a superlattice with artificially designed lattice symmetry under large magnetic fields. A Fstst spectrum in which the lattice symmetry is different from the main crystal. The results of this study establish a stable and diverse technique with dynamic tunability for the energy band structure engineering of graphene and related van der Waals materials. (Nature Nanotechnology DOI: 10.1038/s41565-018-0138-7)
4. Catalytic chemoselective functionalization of methane in metal-organic frameworks
Material Name: Metal Organic Framework Compound
Research Team: Farha Research Group, Northwestern University, USA
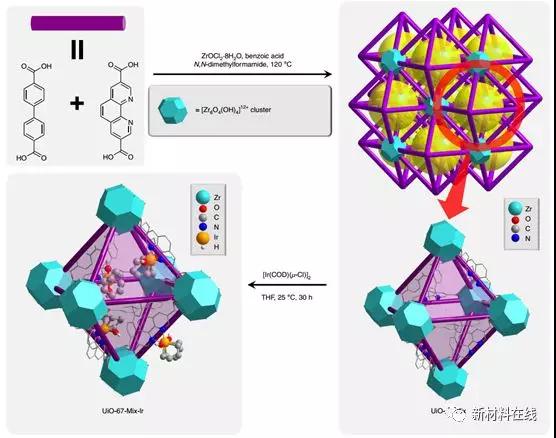
Methane is the largest component of natural gas reserves and is a low-cost, high-reserve raw material for the synthesis of high value-added chemicals and fuels. Because of the low intrinsic activity of methane, its selective catalytic functionalization is an important goal in the chemical sciences. Oxyboration has recently become a promising approach to achieving catalytic functionalization of methane. A major challenge in this regard is the selective boration of monomethylated products, which are more active than methane and tend to cause excessive functionalization. Zhang et al. reported a highly selective microporous metal-organic framework-supported, methane oxyborated ruthenium catalyst with more than 99% chemoselectivity for monooxo-methane (single versus double) The yield was 19.5% and the number of conversions was 67), wherein bis(pinacol borane) was used as the boration reagent in dodecane, and the reaction was carried out in methane at 150 ° C and 34 atm. This tendency of the product monoboration is attributed to the shape selective action of the metal-organic framework pore structure. (Nature Catalysis DOI: 10.1038/s41929-018-0069-6)
5. Control the magnetic properties in 2D CrI3 by electrostatic doping
(Controlling magnetism in 2D CrI3 by electrostatic doping)
Material Name: Two-dimensional CrI3-graphene heterojunction
Research Team: Jie Shan Research Group, Cornell University, USA
The atomic thickness of a two-dimensional material provides a unique opportunity to control its electrical and optical properties and to drive electronic phase transitions by electrostatic doping. The discovery of two-dimensional magnetic materials opens up new prospects for the realization of magnetic electrical control and new functional devices. Recent experiments based on linear magnetoelectric effects have confirmed the ability to control the magnetic sequence in the bilayer CrI3 by an electric field. However, this method is limited to non-centrosymmetric materials that are magnetically biased near the antiferromagnetic-ferromagnetic transition. Jiang et al. demonstrated the use of CrI3-graphene vertical heterostructures for electrostatic doping to control the magnetic properties of single and double layer CrI3. In a single layer of CrI3, doping significantly changes the saturation magnetization, coercivity, and Curie temperature, and exhibits a magnetic sequence that increases/decreases with hole/electron doping. It is worth noting that in the absence of a magnetic field, electron doping above 2.5 × 10 13 · cm -2 in the double-layer CrI3 leads to a transition from an antiferromagnetic ground state to a ferromagnetic ground state. The results reveal a strong inter-layer exchange coupling associated with doping, which enables stable conversion of magnetization in a two-layer CrI3 with a small gate voltage. (Nature Nanotechnology DOI: 10.1038/s41565-018-0135-x)
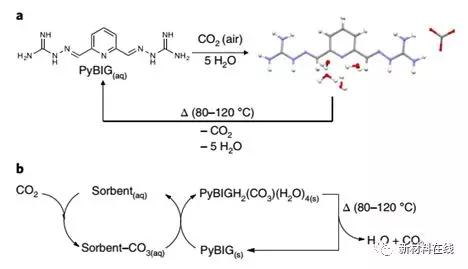
6. Capture carbon dioxide directly from the air
(Direct air capture of CO2 via aqueous-phase absorption and crystalline-phase release using concentrated solar power)
Material Name: Carbonate Crystal
Research Team: Custelcean Research Group, Oak Ridge National Laboratory, USA
The use of negative emission technologies to purify greenhouse gases in the atmosphere provides a way to limit global temperature rise. Direct air capture of carbon dioxide provides a good prospect for permanently reducing atmospheric carbon dioxide concentrations, and can be developed and deployed on a large scale if economical and energy efficient technologies are available. Brethomé et al. report a laboratory-scale direct air collection method that primarily utilizes off-the-shelf materials and equipment. First, CO2 absorption can be achieved by using a household humidifier through an off-the-shelf environmentally friendly amino acid solution (glycine and sarcosine). The solution containing CO2 is then reacted with a simple hydrazine compound (which is a very insoluble carbonate crystal) and an amino acid adsorbent is regenerated. Finally, by using carbonated crystals to be relatively mildly heated using concentrated solar energy, efficient CO2 release and near-quantitative ruthenium compound regeneration can be achieved. (Nature Energy DOI: 10.1038/s41560-018-0150-z)
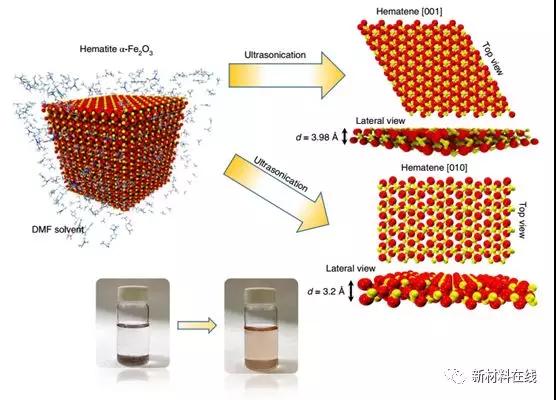
7. Stripping non-Vanderford materials from iron ore hematite
(Exfoliation of a non-van der Waals material from iron ore hematite)
Material Name: Two-dimensional material hematene (hematene)
Research Team: Ajayan Research Group, Rice University, USA
As the most studied graphene in two-dimensional materials, many inorganic analogs have been synthesized and are being developed for new applications. There are a number of methods that can be used to obtain large particles, high quality materials. For example, naturally occurring ores are the best precursors for obtaining highly ordered and large particle atomic layers by exfoliation. Balan et al. demonstrated a new two-dimensional material, hematene (hematene), which is obtained from natural iron ore hematite (α-Fe2O3) separated by liquid stripping. Transmission electron microscopy confirmed the two-dimensional morphology of hematene. The magnetic measurement and density functional theory calculations confirm the ferromagnetic order in hematene, while the relative parent form presents antiferromagnetic order. When loaded on a titania nanotube array, hematene exhibits enhanced visible light photocatalytic activity. This study shows that although the band arrangement is not conducive to charge transfer, photogenerated electrons can be transferred from hematene to titanium dioxide. (Nature Nanotechnology DOI: 10.1038/s41565-018-0134-y)
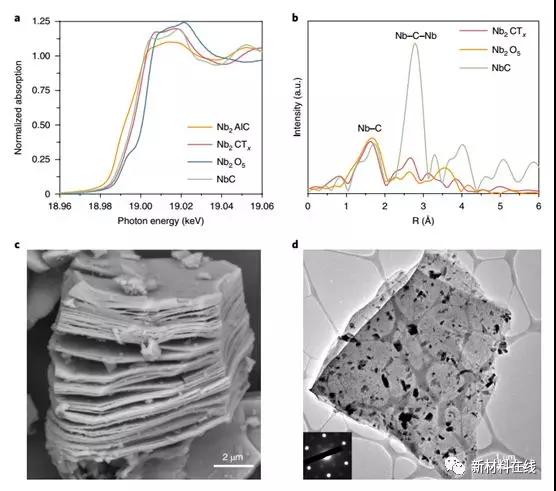
8. Reactive metal-support interaction of two-dimensional 铌-carbide-supported platinum catalyst at moderate temperatures
(Reactive metal–support interactions at moderate temperature in two-dimensional niobium-carbidesupported platinum catalysts)
Material Name: 铌-Carbide Support Platinum Catalyst
Research Team: yue Wu Research Group, Iowa State University, USA
Reactive metal-support interactions (RMSI) provide electronic, geometric, and component effects that can be used to modulate catalytically active sites. In general, carriers other than oxides are not considered candidates for RMSI. Li et al. reported an example of non-oxide-based RMSI between platinum and Nb2CTx MXene, a recently developed two-dimensional metal carbide. The surface functional groups of the two-dimensional carbide can be reduced and the Pt-Nb surface alloy can be formed at a moderate temperature (350 ° C). This alloy exhibits a lower CO adsorption than monometallic platinum. The water gas shift reaction kinetics showed that the RMSI stabilized the nanoparticles and formed an alloy-MXene interface with higher H2O activation ability than the non-reducing carrier or bulk tantalum carbide. The RMSI between platinum and rhodium-MXene supports can be extended to other members of the MXene range and opens up new avenues for the design and operation of functional bimetallic catalysts. (Nature Catalysis DOI: 10.1038/s41929-018-0067-8)
Plastic Grilles,Plastic Return Air Grille,Plastic Air Vent Grilles,Plastic Ventilation Grilles
Guangzhou Jointair Co., Ltd. , https://www.jointairaccessories.com
